The difficulties involved in sharing clinical trial data
News from the Committee
28/06/2023
Several members of the Committee for Open Science’s Clinical trial data sharing statement project have co-authored an article to support the implementation of data sharing.
‘Ten (not so) simple rules for clinical trial data-sharing’ sets outs 10 rules covering the various elements required to successfully complete the sharing process and facilitate the effective re-use of data for new research questions.
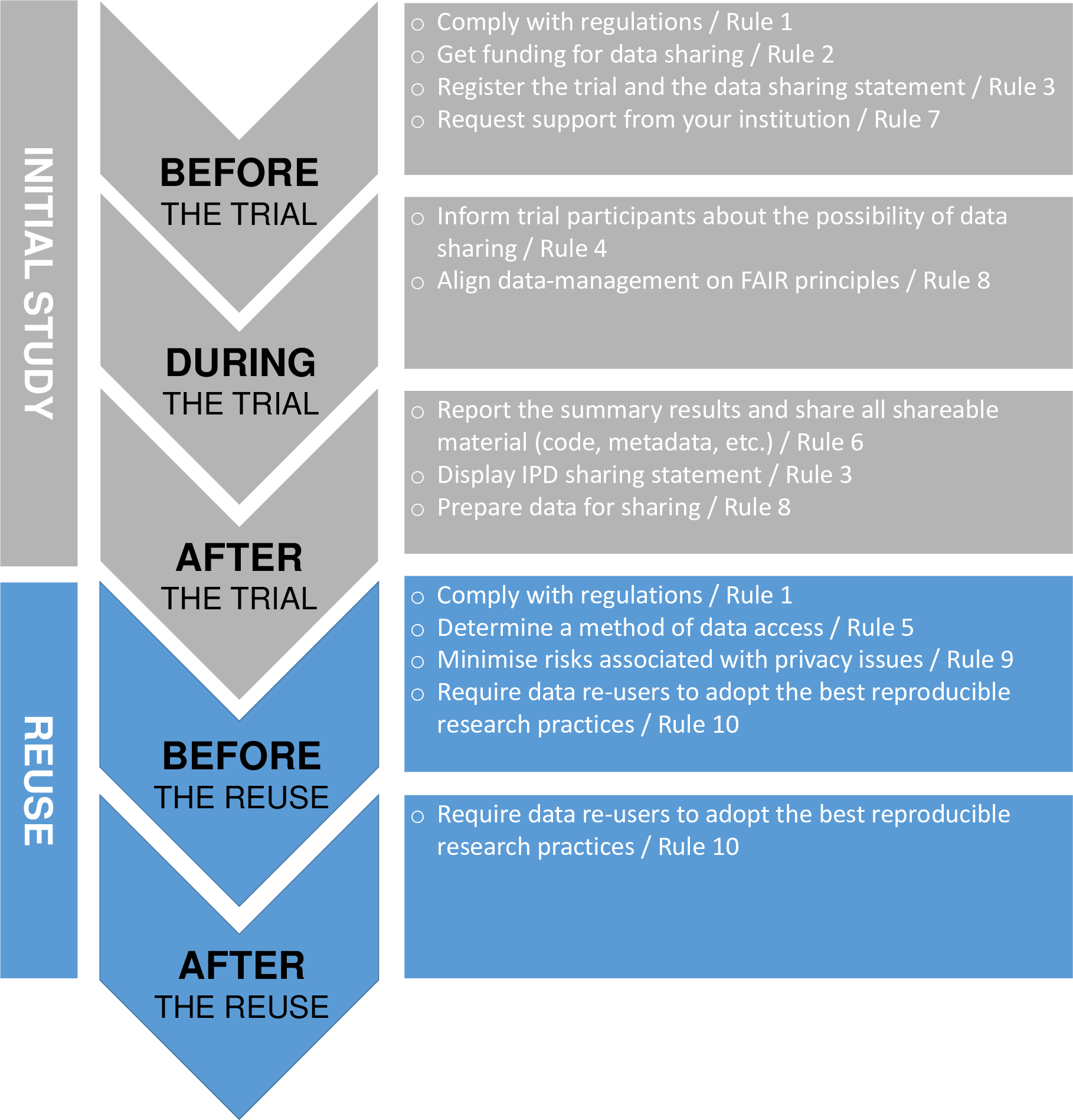
@Pellen et al
The figure above shows the rules aligned with the clinical trial life cycle.

Next post

News from the Committee
22/06/2023
The project The socioeconomics of scientific publication of the French Committee for Open Science has published a study on Diamond open access journals business models. The aim of this study is to test the feasibility, and also the desirability, of…



